Diagnosing Metal Degradation: Erosion Vs. Corrosion
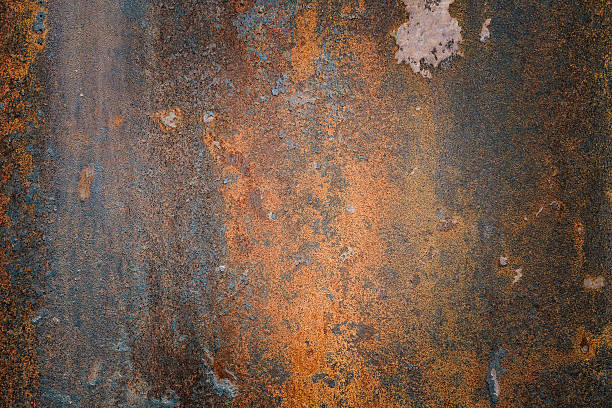
It is crucial for the designers, manufacturers, purchasers, and users of metal parts, tools, devices, and equipment to understand the forces continually at work trying to break them down. This requires a deep understanding of the forces that challenge the integrity of metals, namely erosion and corrosion.
These terms might sound similar, and indeed, their effects can be devastating. However, erosion and corrosion are two different and distinct processes.
The Mystery of Metal Erosion
When we speak of erosion, images of wind and water reshaping landscapes come to mind. It's the process where surface elements like soil or rock are carried away to another location. While occurring on a much smaller scale, metal erosion happens similarly.
Metal erosion occurs when moving liquids or gases interact with a metal's surface, transporting its particles elsewhere. Continuous exposure can reshape the metal, weaken it, and, in due course, lead to its failure. Picture a beachfront gradually eroding due to persistent waves – that's essentially what's happening on a metal surface on a microscopic scale.
Decoding Metal Corrosion
Corrosion is a transformative natural process that converts a refined metal into a more chemically stable form, such as oxide, hydroxide, carbonate, or sulfide. It is the gradual destruction of materials (usually metal) by chemical or electrochemical reactions with their environment. Unlike erosion, which is primarily physical, corrosion is rooted in chemical and electrochemical interactions. Think of rust: it changes the metal's color and makes it more susceptible to physical damage.
Identifying Metal Erosion and Corrosion
When it comes to erosion, the primary impact is a visible alteration in the metal's surface. For instance, a metal exposed to continuous gas flow may exhibit a broad, shallow indentation, while another subjected to liquid flow may develop grooves or holes, depending on the angle of consistent impact. Importantly, even with this reshaping, the affected areas often retain the metal's original color and material characteristics; they're just distorted.
Corrosion, a chemical or electrochemical process, produces more dramatic visual changes. Rust is a good example. A metal piece originally black or gray can turn to a pronounced reddish-brown as corrosion sets in.
Moreover, it's not rare for erosion and corrosion to be co-conspirators. An eroded surface that has lost its protective layer may expose the underlying metal, making it vulnerable to corrosion.
High-Risk Zones for Erosion and Corrosion
While erosion and corrosion can act on the same metal surface, the conditions fostering each differ. Erosion is more prevalent in metals continually in contact with moving liquids and gases. Within systems that handle these substances, specific areas are more vulnerable. For instance, metal subjected to water pumped through pipes will experience less erosion in straight portions. However, where the pipe curves or bends, the erosive forces intensify.
Corrosion, conversely, is a product of specific environments. Areas exposed to chemicals, acids, oxides, and other reactive agents are corrosion hotspots. Notably, these chemicals don't need to be in motion to instigate corrosion. Their mere presence and interaction with metals, a dance of electrons, can cause degradation.
Furthermore, there's the concept of erosion-corrosion. In this phenomenon, the corrosion process is accelerated due to the relative movement of the corrosive substance and the metal surface.
Defense Against Erosion and Corrosion
Though distinct, erosion and corrosion can be mitigated by a common solution: protective coatings. Chromium, recognized for its exceptional hardness, offers an effective defense. Coating metals with a thin chromium layer can extend their life significantly, making it harder for erosive forces to strip the material away and for corrosive reactions to occur. For manufacturers and users of metal components, chromium coating can greatly extend the durability and efficiency of their products.
In the dynamic field of manufacturing, understanding and countering these forces is crucial. Adopting proactive measures like chromium coating can be the differentiator in the product's lifecycle, ensuring optimal performance and longevity.
